Propofol, Nebulized Dexmedetomidine and a Surprising Epilepsy Lead - What’s Next for the Anesthesia Drugs Market?
Published: 2025-09-16
Lede - Three recent research threads - a deeper look at how propofol causes unconsciousness, promising results for nebulized dexmedetomidine in pediatric GI endoscopy, and early-stage work repurposing an anesthetic for epilepsy - together point to meaningful shifts in clinical practice, R&D priorities, and commercial strategy across the anesthesia drugs market.
What the Studies Found
-
Propofol and brain dynamics: New mechanistic work suggests propofol doesn’t just “slow” the brain - it pushes neural networks out of a fragile balance between responsiveness and chaos, producing loss of consciousness through altered dynamic stability. That framing could change how we monitor and titrate anesthesia.
Researchers studied how propofol affects brain activity in animals by recording electrical signals in areas related to vision, hearing, spatial awareness, and executive function. Using a technique called delay embedding, they tracked how the brain responds to sensory inputs. Normally, neural activity spikes then quickly return to baseline, but under propofol, the brain stayed in an overly excited state, becoming increasingly unstable until consciousness was lost.
A computational model showed the same effect: increasing inhibition destabilized neural networks due to disinhibition, where inhibitory neurons suppress other inhibitory neurons, paradoxically boosting overall activity. The team suggests other anesthetics might cause unconsciousness through similar mechanisms.
These insights could improve anesthesia control systems, allowing precise, real-time dosage adjustments, and the method may also be applied to studying other brain states and neuropsychiatric disorders.
-
Nebulized dexmedetomidine for kids: Early clinical reports show nebulized dexmedetomidine may be an effective, well-tolerated, non-invasive sedative for pediatric GI procedures - especially useful when IV access is difficult or distressing.
A recent study highlights the effectiveness of nebulized dexmedetomidine in pediatric patients undergoing upper gastrointestinal endoscopy. Key findings include:
-
Significantly reduced gag reflex and coughing compared to the control group.
-
Fewer limb movements against the endoscope, improving procedure ease.
-
Lower parental separation anxiety, with more children experiencing easy separation.
-
Reduced requirement for additional sedative agents, leading to higher endoscopist satisfaction.
-
Recovery times were similar between groups, indicating safety and no prolonged sedation.
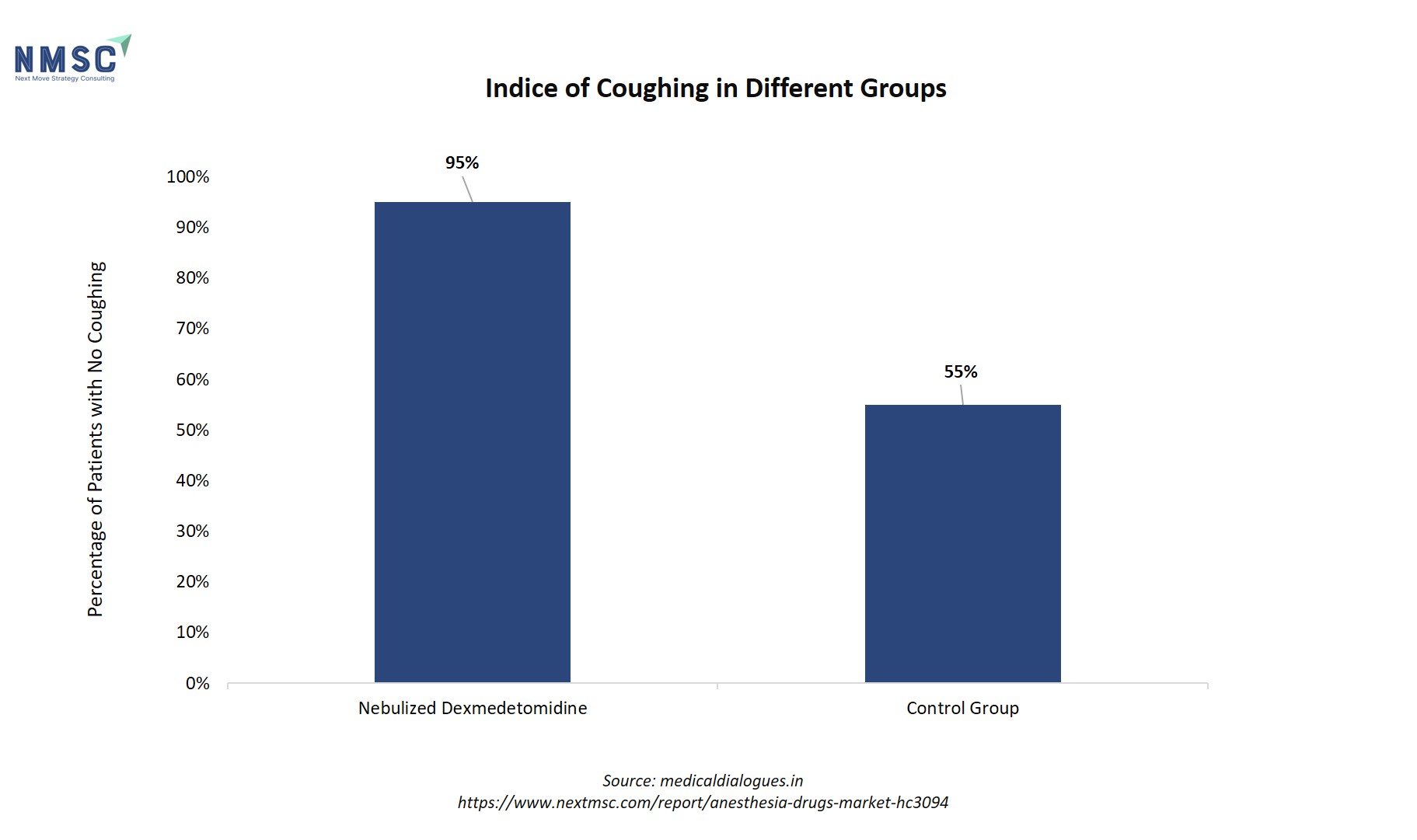
The study revealed a significant difference between the group receiving the treatment and the control group. The accompanying bar chart visually captures this outcome, showing that patients treated with dexmedetomidine experienced substantially less coughing. Specifically, 95% of patients in the dexmedetomidine group had no coughing, compared to only 55% in the control group, underscoring the treatment's potent cough-suppressant effect.
-
Repurposing anesthetics for epilepsy: Exploratory drug-discovery work indicates certain anesthetic compounds may have anti-seizure potential. If validated, this opens the door to new therapeutic uses beyond the OR.
Nazzareno Avanzo, a biophysicist at the University of Montreal and an ion channel expert not involved in the study, called the findings “a really big step” and said they solved a problem previously thought unsolvable. He added that the study was particularly notable because it not only identified a potential binding site for a known anesthetic drug but also showed that the drug could rescue disease-causing mutations—something he had not heard of before.
The research opens possibilities for using propofol to treat HCN1-related epilepsy and for discovering other molecules without propofol’s sedative side effects. “Propofol is a nice molecule, in terms of helping people, because it is already FDA-approved,” said Nimigean. Avanzo also noted that the findings “have the potential to lead to the development of more specific treatments for patients with these epileptic mutations, ushering in a new era of structural biology.”
Next Move Strategy Consulting - What This Means (Opinion & Impact)
These three developments are distinct, but together they point to strategic moves companies and health systems should be thinking about now:
-
Monitoring and software will matter more than ever: If anesthetic action is best described in terms of changing network dynamics, single-value monitors (simple BIS numbers etc.) look limited. There’s an accelerating case for richer, algorithmic monitoring and drug+device combos that can run closed-loop dosing or offer real-time neural-state feedback. Drug makers who partner with monitor/software vendors will have an edge.
-
Formulation & delivery routes are a low-friction growth lever: Nebulized and non-IV delivery expands where sedation can be given (outpatient clinics, mobile units, pediatric centers) and improves patient experience. Investing in alternative formulations and the IP around them is often cheaper and faster than inventing entirely new molecules - and it widens the set of buyers (ambulatory care, pediatrics).
-
Repurposing lowers development time - but needs clinical proof: Repurposing anesthetics for neurology or other CNS disorders can leverage existing safety data and manufacturing know-how, potentially speeding development. The commercial upside is meaningful, but only if efficacy in the new indication is robust and durable. Expect collaborations between academic groups and small biotechs, with larger pharma stepping in at later stages.
-
R&D will shift toward selectivity and pairing with monitoring: Mechanistic insights argue for drugs that modulate circuits more precisely - receptor-subtype selectivity, allosteric modulators, or molecules designed to be used with neuromonitoring/algorithms. This reduces side-effect risk and makes dose management smarter, which clinicians and payors will value.
-
Adoption needs guideline and training changes: New delivery routes and new indications require clinical guidance, training, and real-world evidence. Stakeholders who invest in clinician education and early post-market studies will shorten the adoption curve.
Practical Implications for Stakeholders
-
Hospitals & clinics: Pilot alternative sedation pathways (e.g., nebulized approaches) in low-risk outpatient settings and evaluate richer monitoring solutions in high-volume ORs.
-
Pharma & biotech: Prioritize formulation IP (nebulized/mucosal) and explore repurposing candidates where safety dossiers already exist. Consider co-development with device/monitoring partners.
-
Investors: Look for companies combining drugs and monitoring tech - bundled solutions reduce clinical uncertainty and increase switching costs.
-
Regulators & payors: Expect requests for post-market surveillance and real-world effectiveness data for new routes and repurposed uses.
Timing & Caveats
Promising mechanistic studies and small clinical reports do not guarantee rapid market change. Larger confirmatory trials, cost-effectiveness studies, and regulatory clarity are needed before these trends translate into broad commercial shifts. Repurposing can be faster because of existing safety data, but efficacy in new disease areas remains the gating factor.
Conclusion
Recent research nudges the field in three overlapping directions: better mechanistic understanding that favors smarter monitoring, non-IV delivery that broadens care settings (especially for pediatrics), and repurposing that could expand the clinical footprint of anesthetic molecules. For the anesthesia drugs market, the next several years are likely to be defined less by unit price wars and more by who can pair safe, selective chemistry with intelligent delivery and monitoring - and who can prove those combinations improve outcomes and workflow in real world settings.
About Next Move Strategy Consulting:
Next Move Strategy Consulting is a premier market research and management consulting firm that has been committed to provide strategically analysed well documented latest research reports to its clients. The research industry is flooded with many firms to choose from, what makes Next Move different from the rest is its top-quality research and the obsession of turning data into knowledge by dissecting every bit of it and providing fact-based research recommendation that is supported by information collected from over 500 million websites, paid databases, industry journals and one on one consultations with industry experts across a diverse range of industry sectors. The high-quality customized research reports with actionable insights and excellent end-to-end customer service help our clients to take critical business decisions that enables them to move beyond time and have competitive edge in the industry.
We have been servicing over 1000 customers globally that includes 90% of the Fortune 500 companies over a decade. Our analysts are constantly tracking various high growth markets and identifying hidden opportunities in each sector or the industry. We provide one of the industry’s best quality syndicate as well as custom research reports across 10 different industry verticals. We are committed to deliver high quality research solutions in accordance to your business needs. Our industry standard delivery solutions that ranges from the pre consultation to after-sales services, provide an excellent client experience and ensure right strategic decision making for businesses.
For more information please contact:
Next Move Strategy Consulting
5th Floor 867
Boylston St, STE 500,
Boston, MA 02116, U.S.
E-Mail: [email protected]
Direct: +18577585017
Website: www.nextmsc.com
About the Author
 Joydeep Dey is an SEO Executive, Content Writer, and AI enthusiast with over 2½ years of experience in digital marketing and artificial intelligence. He specializes in crafting smart SEO strategies, creating engaging content, and exploring AI-powered solutions that boost online visibility and audience engagement. Outside of work, you’ll often find Joydeep exploring new gadgets, binge-watching a good series, or just wandering around for fresh inspiration.
Joydeep Dey is an SEO Executive, Content Writer, and AI enthusiast with over 2½ years of experience in digital marketing and artificial intelligence. He specializes in crafting smart SEO strategies, creating engaging content, and exploring AI-powered solutions that boost online visibility and audience engagement. Outside of work, you’ll often find Joydeep exploring new gadgets, binge-watching a good series, or just wandering around for fresh inspiration.
About the Reviewer
 Sanyukta Deb is a seasoned Content Writer and Team Leader in Digital Marketing, known for her expertise in crafting online visibility strategies and navigating the dynamic digital landscape. With a flair for developing data-driven campaigns and producing compelling, audience-focused content, she helps brands elevate their presence and deepen user engagement. Beyond her professional endeavors, Sanyukta finds inspiration in creative projects and design pursuits.
Sanyukta Deb is a seasoned Content Writer and Team Leader in Digital Marketing, known for her expertise in crafting online visibility strategies and navigating the dynamic digital landscape. With a flair for developing data-driven campaigns and producing compelling, audience-focused content, she helps brands elevate their presence and deepen user engagement. Beyond her professional endeavors, Sanyukta finds inspiration in creative projects and design pursuits.
















Add Comment