Global Vaccines Market Anchored by Major Donations and Regulatory Shifts
Published: 2025-08-12

The vaccines landscape in 2024–2025 has been shaped by large scale dose donations to outbreak zones and significant policy changes in clinical trial requirements. Two developments—Canada’s donation of mpox vaccines to the Democratic Republic of Congo and the U.S. Department of Health and Human Services’ new placebo testing mandate—illustrate the evolving dynamics of supply, access and regulatory rigor.
Latest Developments in Vaccine Development, Testing, and Regulation
-
Traditional vaccine development from pathogen identification to public use averages 10–15 years.
-
Exploratory research (2–4 years) discovers candidate antigens; preclinical testing (1–2 years) uses lab models and in silico simulations.
-
IND applications prompt a 30 day FDA safety review before clinical trials.
-
Phase I (20–80 adults), Phase II (hundreds), and Phase III (thousands) trials verify safety, dosing, and efficacy.
-
Post marketing surveillance through VAERS, V Safe, VSD, and CISA monitors safety in real world settings.
-
Emergency acceleration, exemplified by Operation Warp Speed, overlapped trial phases and pre licensure manufacturing to compress timelines.
Canada–Gavi Delivers 200,000 Mpox Vaccine Doses to DRC
On 11 February 2025, a shipment of 200,000 mpox vaccine doses donated by the Government of Canada and facilitated by Gavi, the Vaccine Alliance, arrived in Kinshasa, Democratic Republic of the Congo, under the Access and Allocation Mechanism for mpox. These doses raise the total number of mpox vaccines allocated by Gavi from various donors to 1,215,460, alongside an additional 500,000 doses funded directly by Gavi itself. Canada’s Minister of International Development, Ahmed Hussen, noted that this contribution builds on Canada’s US $1 million commitment to the World Health Organization’s mpox response in the region and will “help stop the spread of the virus and get vaccines to those who need them most”. Gavi CEO Dr Sania Nishtar added that the donation “comes at a time when the DRC is refining its vaccination strategy to ensure greater reach and impact among affected communities.
U.S. to Require Placebo Testing for All New Vaccines
On May 1, 2025, the U.S. Department of Health and Human Services announced that every new vaccine—and even updated versions of existing vaccines—will now be required to undergo placebo controlled trials before receiving approval. HHS Secretary Robert F. Kennedy Jr. called the policy “a radical departure from past practices” in an interview with the Washington Post. Mansoor Amiji, chair of Pharmaceutical Sciences at Northeastern University, cautioned that this mandate could push back availability of updated COVID 19 shots, since placebo trials often run for months or even years despite extensive prior safety data. Novavax has already indicated that its submission for an updated COVID 19 vaccine has been put on hold pending completion of a “full fledged clinical trial,” per the new HHS requirements.
Key Players & Recent Strategic Deals
-
Biofarma
In November 2024, Biofarma teamed up with the Bill & Melinda Gates Foundation to scale production of the novel oral poliovirus vaccine type 2 (nOPV2) in Indonesia. This alliance aims to accelerate global polio eradication by expanding manufacturing capacity and ensuring affordable supply in high risk regions.
-
Bharat Biotech International Ltd.
-
September 2024: Entered a co development and commercialization agreement with Alopexx Inc. to advance AV0328, an investigational antimicrobial vaccine, across India and select low income countries—broadening its infectious disease portfolio.
-
August 2024: Partnered with Hilleman Laboratories to launch Hillchol (BBV131), the first single strain oral cholera vaccine. This collaboration addresses the global cholera vaccine shortage by combining Bharat Biotech’s scale with Hilleman’s formulation expertise.
-
-
BioNTech SE
In August 2024, BioNTech initiated a Phase I clinical trial of an mRNA based therapy targeting non small cell lung cancer (NSCLC), leveraging its COVID 19 vaccine platform to pioneer oncology applications. This move underscores BioNTech’s strategic pivot into therapeutic vaccines.
-
Merck & Co., Inc.
In April 2024, Merck expanded its German research campus to bolster antibody manufacturing R&D. This facility enlargement is part of Merck’s broader strategy to enhance biologics pipeline throughput and support next generation vaccine platforms.
-
Indian Immunologicals Ltd.
In January 2024, Indian Immunologicals launched Havisure, its proprietary Hepatitis A vaccine, following successful clinical validation. This product introduction strengthens India’s domestic vaccine offerings and addresses regional hepatitis A burdens.
Future Prospects
-
Ongoing assessments will determine whether the placebo testing mandate applies solely to emerging disease vaccines or also to chronic disease platforms.
-
Bioscience stakeholders will evaluate the trade off between data robustness and expedited access, particularly for annual influenza and COVID 19 boosters.
-
The mpox donation model may inform future outbreak response frameworks, though replication specifics await further announcements.
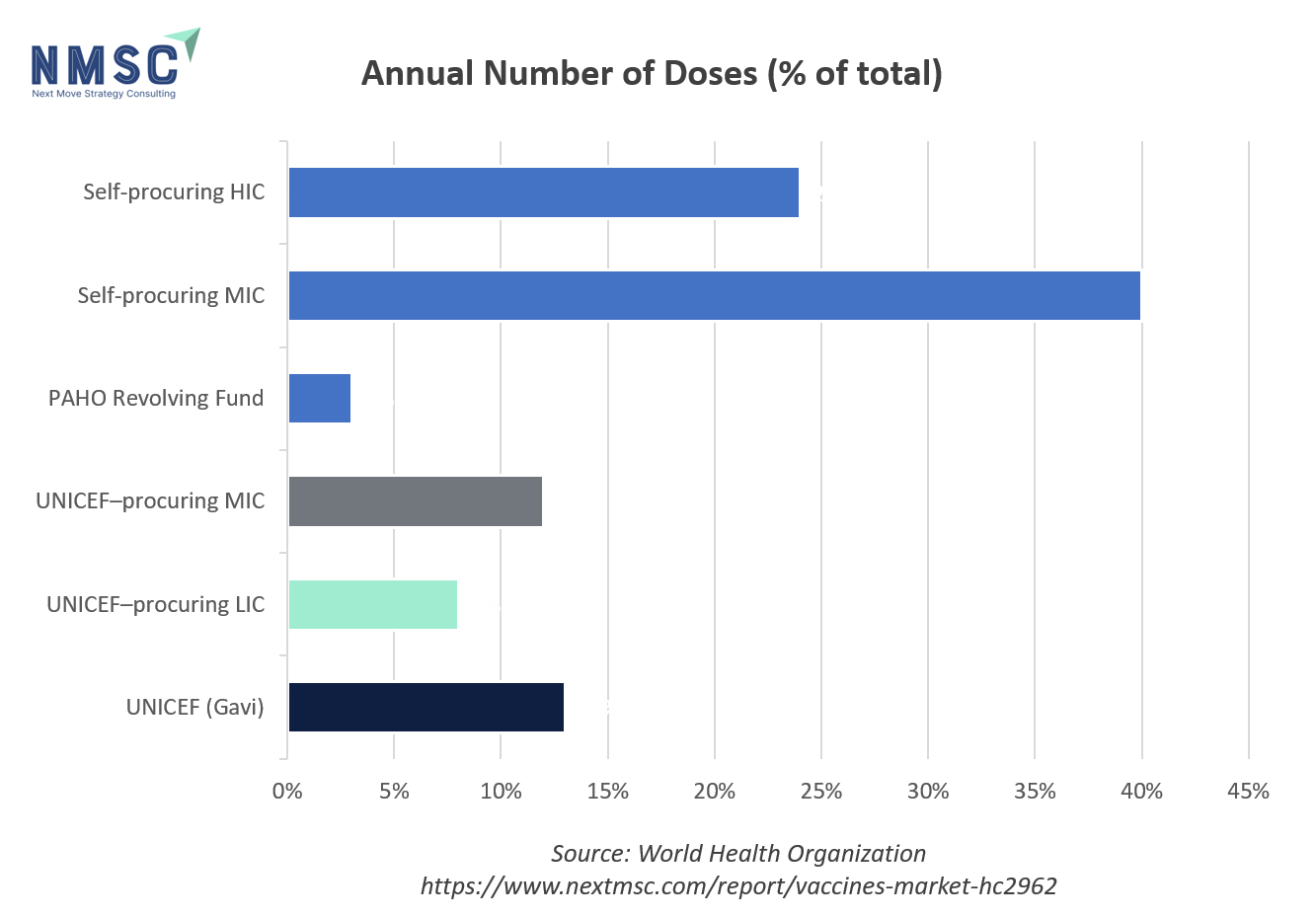
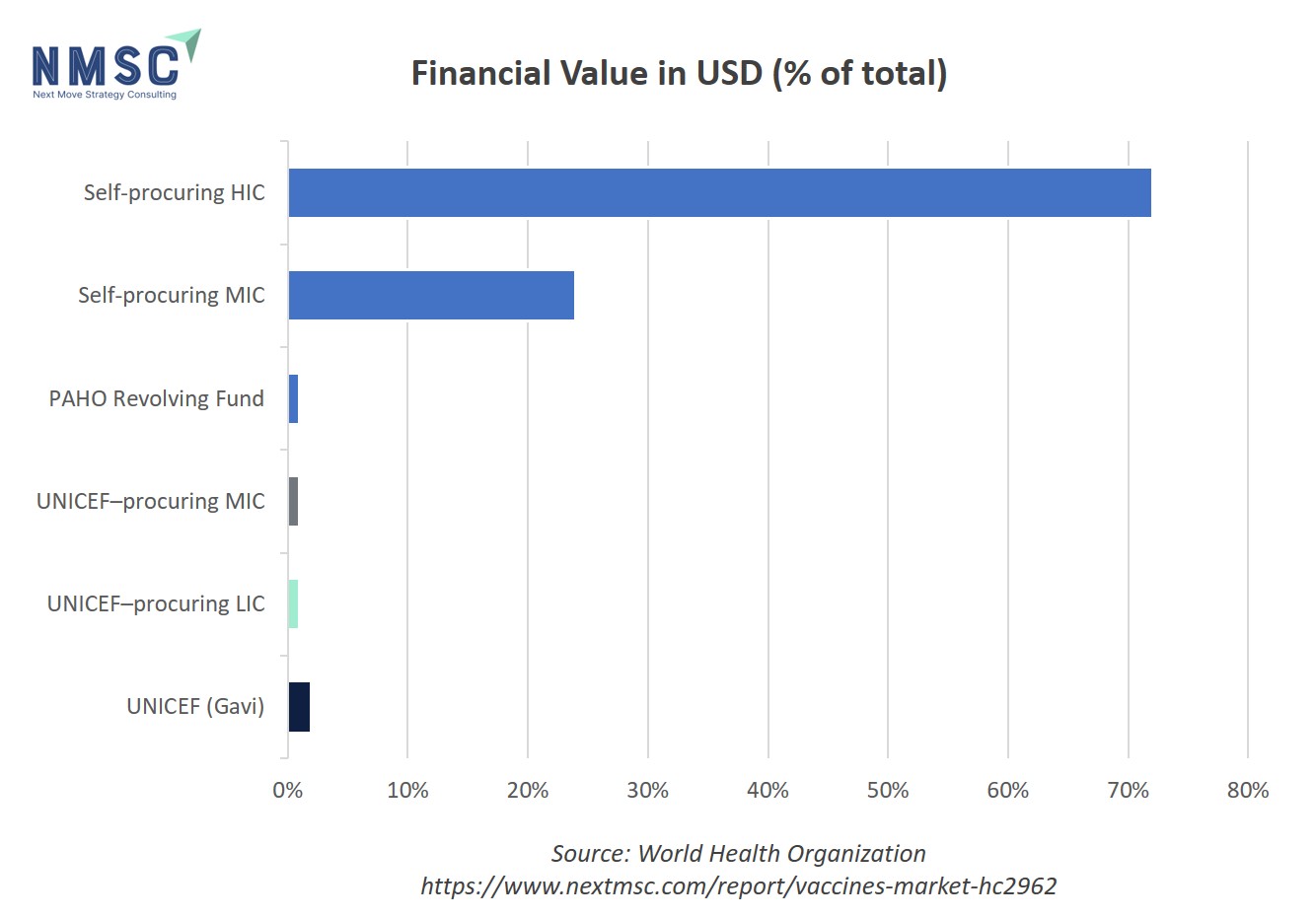
WHO Vaccine Procurement by Volume and Value
In 2023, self‑procuring middle‑income countries led in sheer vaccine volume—accounting for 40 % of all doses, with India and China alone contributing 42 % and 21 % of that segment—while self‑procuring high‑income countries supplied 24 % of doses. UNICEF’s Gavi channel delivered 13 % of doses, followed by UNICEF‑procuring lower‑income and middle‑income countries at 8 % and 12 %, and the PAHO Revolving Fund at just 3 %. Financially, self‑procuring high‑income countries dominated with 72 % of total spend (the U.S. representing 49 % of that), and self‑procuring MICs contributed 24 % of value. By contrast, UNICEF (Gavi) and other pooled‑procurement mechanisms each accounted for only 1–2 % of the financial pie.
About Next Move Strategy Consulting:
Next Move Strategy Consulting is a premier market research and management consulting firm that has been committed to provide strategically analysed well documented latest research reports to its clients. The research industry is flooded with many firms to choose from, what makes NextMSC different from the rest is its top-quality research and the obsession of turning data into knowledge by dissecting every bit of it and providing fact-based research recommendation that is supported by information collected from over 500 million websites, paid databases, industry journals and one on one consultations with industry experts across a diverse range of industry sectors. The high-quality customized research reports with actionable insights and excellent end-to-end customer service help our clients to take critical business decisions that enables them to move beyond time and have competitive edge in the industry.
We have been servicing over 1000 customers globally that includes 90% of the Fortune 500 companies over a decade. Our analysts are constantly tracking various high growth markets and identifying hidden opportunities in each sector or the industry. We provide one of the industry’s best quality syndicate as well as custom research reports across 10 different industry verticals. We are committed to deliver high quality research solutions in accordance to your business needs. Our industry standard delivery solutions that ranges from the pre consultation to after-sales services, provide an excellent client experience and ensure right strategic decision making for businesses.
For more information, please contact:
Next Move Strategy Consulting
5th Floor 867
Boylston St, STE 500
Boston, MA 02116, U.S.
E-Mail: [email protected]
Direct: +18577585017
Website: www.nextmsc.com















Add Comment