Advances in Lung Cancer Diagnostics 2025
Published: 2025-10-04
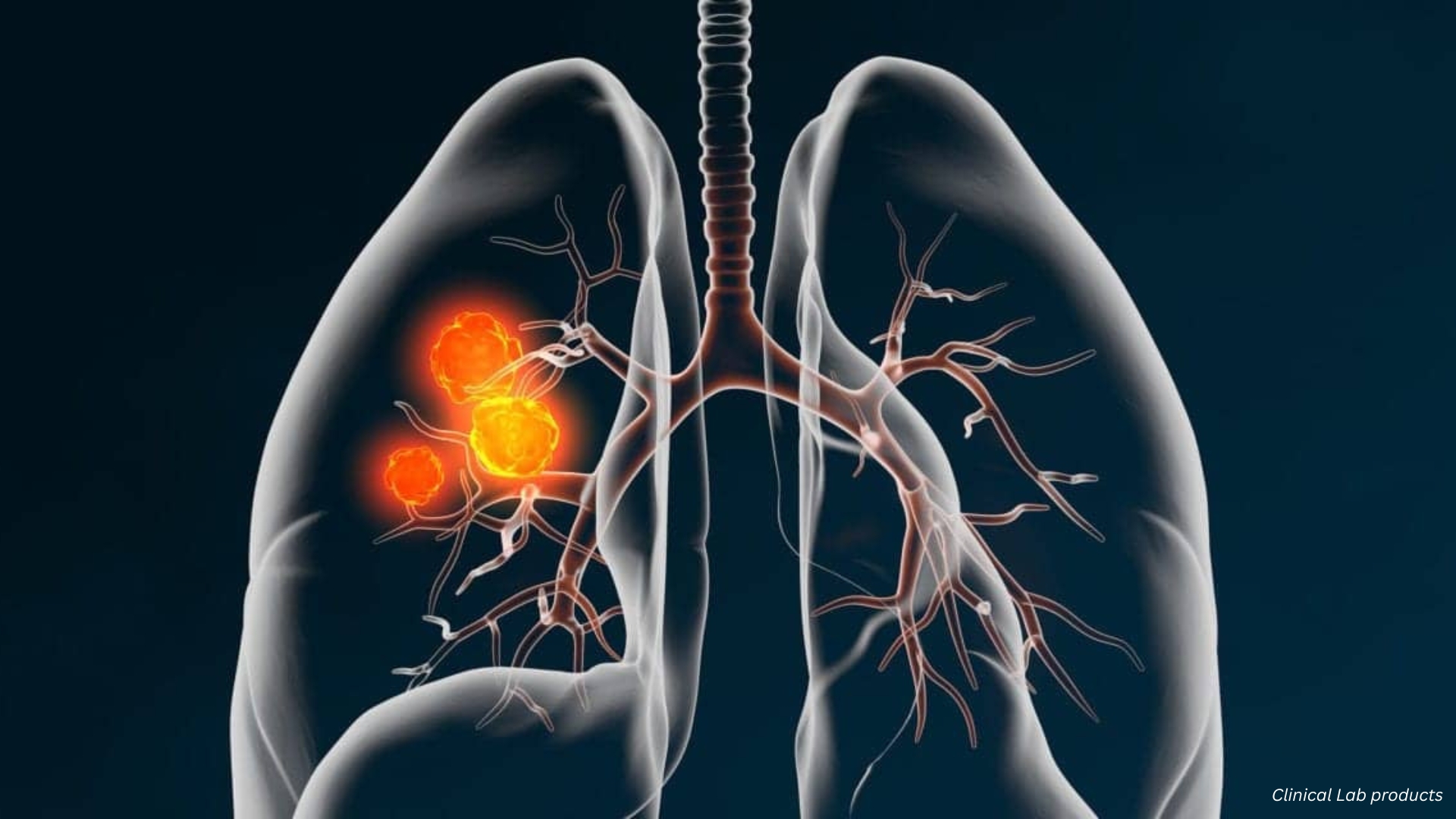
Lung Cancer Diagnostics in 2025: Noninvasive Innovations and Rapid Detection
Lung cancer continues to be one of the leading causes of cancer-related deaths globally. Early detection and precise diagnostics are crucial for effective treatment. In 2025, innovations in lung cancer diagnostics are transforming patient care, making testing less invasive, faster, and more accurate.
Breakthroughs in Noninvasive Diagnostics
One of the most significant advancements in 2025 is the development of CyPath® Lung, a noninvasive diagnostic tool by bioAffinity Technologies. Recently, the Canadian patent office awarded a patent for this technology, recognizing its potential to revolutionize lung cancer screening.
Key Features of CyPath® Lung
-
Noninvasive Procedure: Uses oral and nasal samples instead of tissue biopsies.
-
Early Detection: Capable of identifying cancer at earlier stages, improving treatment outcomes.
-
High Accuracy: Reduces false positives and false negatives common in traditional methods.
Noninvasive diagnostics like CyPath® Lung offer safer, quicker, and more patient-friendly testing options.
Market Leaders Driving Innovation in Lung Cancer Testing
Leading companies in the lung cancer diagnostics industry include Roche Holding AG, Thermo Fisher Scientific Inc., Abbott Laboratories, QIAGEN N.V., Bio-Rad Laboratories, Inc., Hologic Inc., Siemens Healthineers, Genomic Health Inc., Sysmex Corporation, Agilent Technologies, Inc., and others. These industry players are employing strategies such as strategic partnerships and new product launches to sustain their market leadership and drive growth in a competitive landscape.
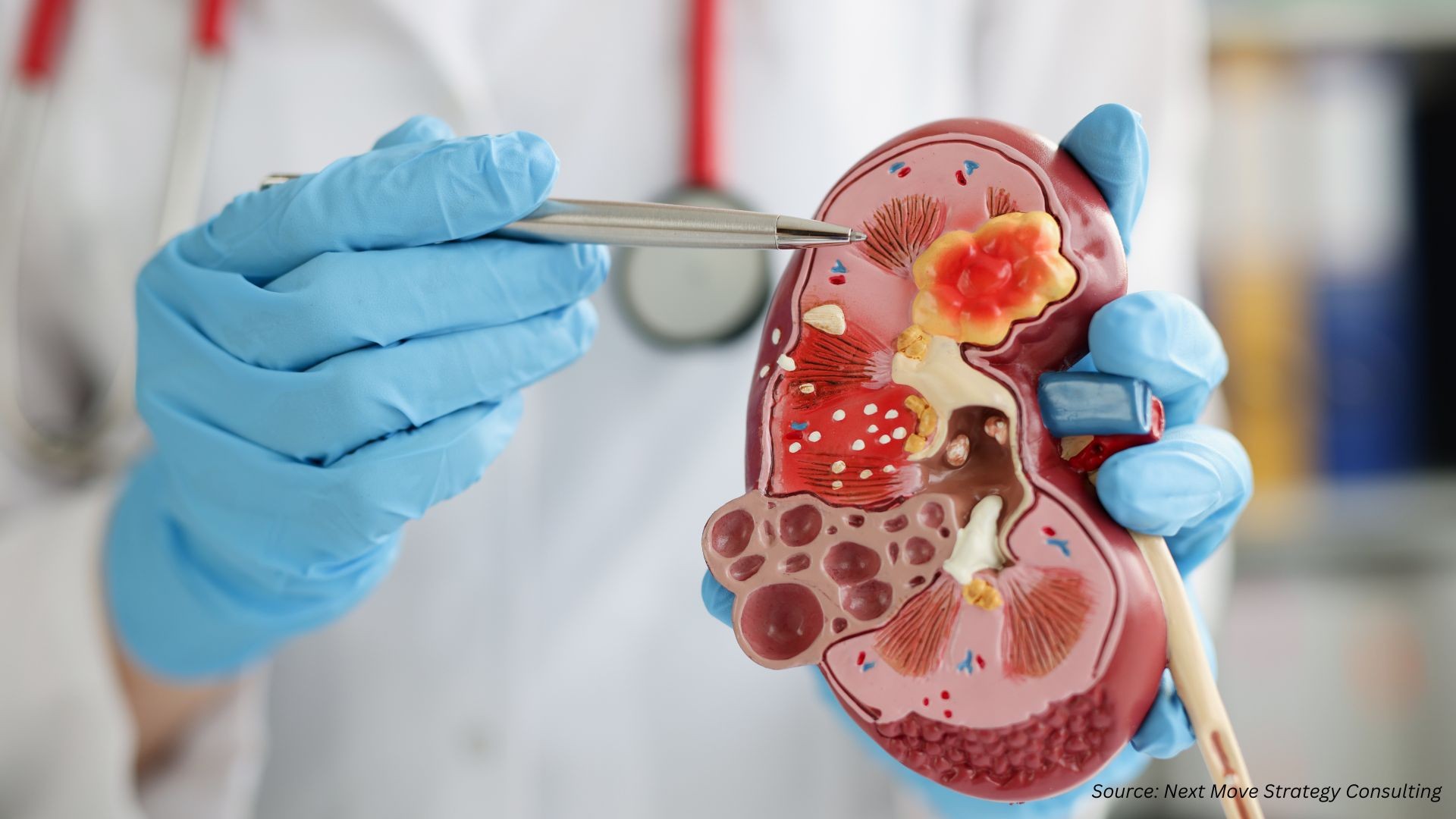
Real-World Implications: Case Study
Dr. D. Ross Camidge, a leading lung cancer researcher, was diagnosed with advanced lung cancer in 2022. His experience underscores the importance of rapid and accurate diagnostics. Within four days, he received a full diagnostic workup including chest x-ray, CT scans, bronchoscopy, biopsy, and molecular testing. This expedited timeline enabled him to begin targeted therapy immediately, demonstrating that fast, coordinated diagnostics can significantly impact patient outcomes.
Key Takeaways from Dr. Camidge's Case:
-
Coordinated diagnostics can reduce waiting times from weeks to days.
-
Molecular testing allows for personalized, targeted therapies.
-
Early and precise detection directly influences survival rates and quality of life.
Traditional vs. Emerging Diagnostic Methods
|
Diagnostic Method |
Description |
Pros |
Cons |
|
CT scan |
Imaging to detect |
Widely available, noninvasive |
Radiation exposure, may miss |
|
Biopsy |
Tissue sample for |
Gold standard for confirmation |
Invasive, potential |
|
CyPath® Lung |
Noninvasive oral/nasal |
Early detection, high accuracy, |
New technology, availability |
Advances in Rapid Molecular Testing
Rapid molecular testing is gaining prominence. Genetic profiling identifies specific mutations driving cancer growth, enabling targeted therapies. In Dr. Camidge’s case, identifying the genetic change in tumor cells allowed him to start therapy within days.
Benefits of Rapid Molecular Testing:
-
Enables personalized medicine
-
Shortens time to treatment initiation
-
Improves patient outcomes
Next Steps: Actionable Takeaways
-
Healthcare Providers: Integrate noninvasive and molecular diagnostics into standard screening protocols.
-
Patients: Seek early testing if at risk, and inquire about noninvasive options like CyPath® Lung.
-
Policy Makers: Support funding and policies to improve accessibility to advanced diagnostic tools.
-
Researchers: Continue validating AI-assisted imaging and noninvasive diagnostics for wider adoption.
-
Public Awareness: Educate communities on the importance of early detection and new testing options.
Next Move Strategy Consulting’s View
Next Move Strategy Consulting views the 2025 advancements in lung cancer diagnostics as a transformative shift in patient care. Innovations such as noninvasive methods like CyPath® Lung, rapid molecular profiling, and AI-assisted imaging are enabling earlier detection, faster treatment initiation, and personalized therapy. It emphasizes that these technologies reduce patient anxiety and procedural risks, improve hospital workflow efficiency, and represent strategic opportunities for healthcare providers to enhance patient outcomes.
Conclusion
Overall, early, accurate, and patient-friendly diagnostics are actively reshaping lung cancer care. Noninvasive testing, rapid molecular analysis, and AI support are setting a new standard for timely, effective, and personalized treatment. While challenges such as accessibility, cost, and regulatory approval remain, the integration of these innovations is driving a new era in lung cancer diagnostics, promising improved survival rates, better quality of life, and more efficient healthcare delivery in 2025 and beyond.
About the Author
 Tania Dey is an experienced Content Writer specializing in digital transformation and industry-focused insights. She crafts impactful, data-driven content that enhances online visibility, and aligns with emerging market trends. Known for simplifying complex concepts. Tania Dey delivers clear, engaging narratives that empower organizations to stay ahead in a competitive digital landscape.
Tania Dey is an experienced Content Writer specializing in digital transformation and industry-focused insights. She crafts impactful, data-driven content that enhances online visibility, and aligns with emerging market trends. Known for simplifying complex concepts. Tania Dey delivers clear, engaging narratives that empower organizations to stay ahead in a competitive digital landscape.
About the Reviewer
 Sanyukta Deb is a skilled Content Writer and Digital Marketing Team Leader, specializing in online visibility strategies and data-driven campaigns. She excels at creating audience-focused content that boosts brand presence and engagement, while also pursuing creative projects and design interests
Sanyukta Deb is a skilled Content Writer and Digital Marketing Team Leader, specializing in online visibility strategies and data-driven campaigns. She excels at creating audience-focused content that boosts brand presence and engagement, while also pursuing creative projects and design interests















Add Comment