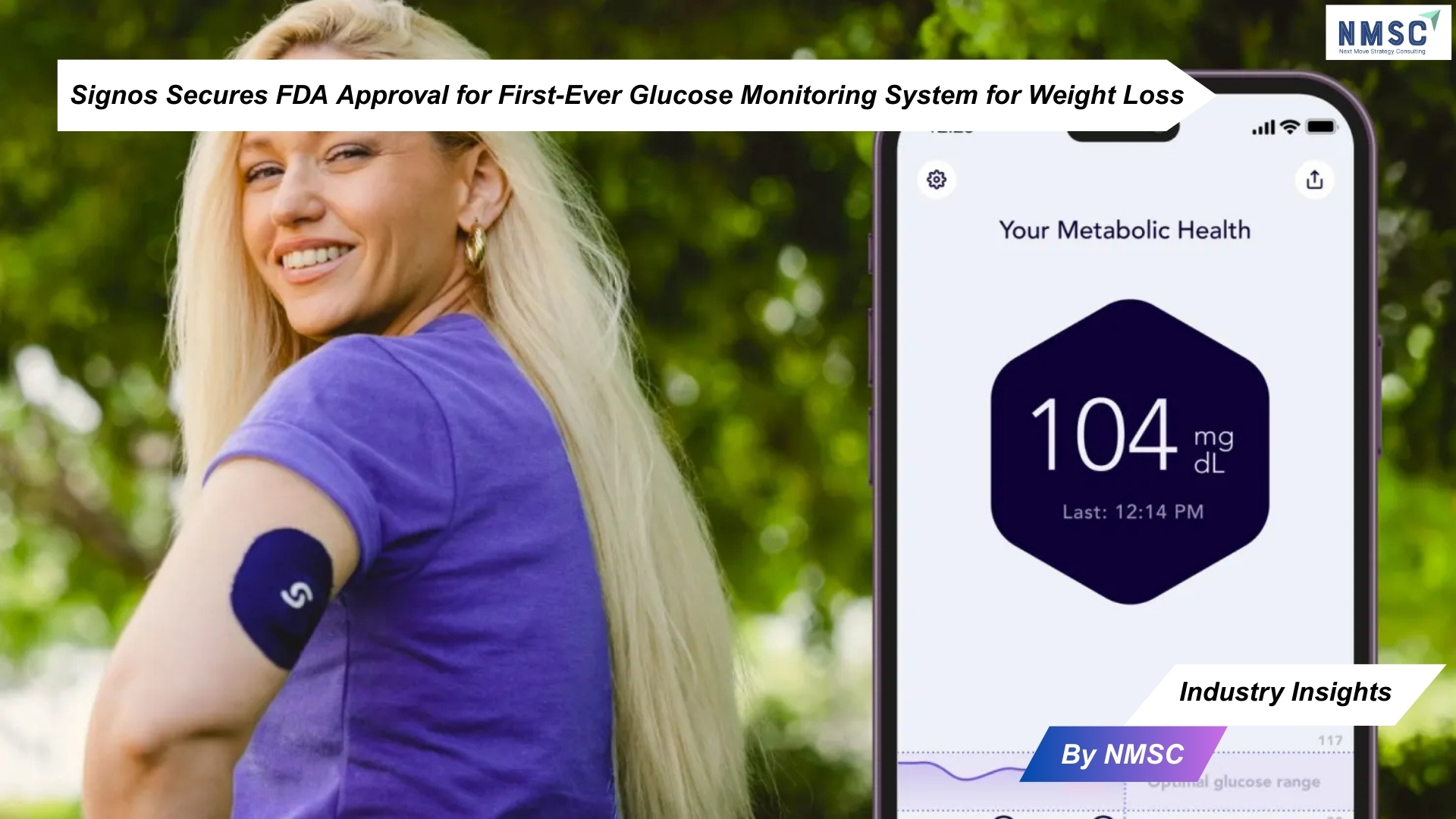
Signos Secures FDA Approval for First-Ever Glucose Monitoring System for Weight Loss
Published: 2025-08-20
Industry Insights from Next Move Strategy Consulting
In a milestone decision for digital health innovation, the U.S. Food and Drug Administration (FDA) has approved the first glucose monitoring (CGM) system designed specifically for weight management. Developed by health-tech startup Signos, the platform combines artificial intelligence with an off-the-shelf CGM from Dexcom to deliver personalized, real-time recommendations for healthier living.
Expanding Weight Management Options
For years, weight-loss interventions have centered on prescription medications such as GLP-1 drugs or invasive approaches like bariatric surgery—options often restricted to patients meeting strict clinical thresholds. By contrast, the FDA’s authorization of Signos opens access to any adult consumer seeking tools for weight maintenance.
“Now there is a solution that everybody can use to help on the weight loss journey,” noted Sharam Fouladgar-Mercer, co-founder and CEO of Signos, underscoring the system’s availability for individuals at varying stages of weight management.
How the System Works
The Signos platform integrates a Dexcom CGM with a mobile app that captures glucose data around the clock. Users can log food intake, activity levels, and lifestyle habits, while the AI-driven software analyzes these inputs to provide actionable guidance. By connecting glucose responses to diet and exercise patterns, the system empowers users to make adjustments that promote sustained weight control.
Signos memberships are available through three- or six-month subscription plans, priced at $139 and $129 per month respectively. Each plan includes all necessary CGMs for the selected duration, shipped directly to customers.
Complementing Existing Treatments
The system is designed to work in tandem with established weight-loss strategies. Patients using GLP-1 medications or those who have undergone bariatric surgery can integrate Signos for ongoing glucose insights. It can also be deployed post-treatment to help maintain progress and reinforce healthier routines.
A Scalable Approach to a National Challenge
With nearly three-quarters of Americans classified as overweight or obese, the economic and health burden of obesity is estimated at more than $170 billion annually. Signos aims to contribute meaningfully to reversing this trend, with its leadership noting the company has already scaled inventory and software infrastructure to meet expected demand following FDA approval.
Market Outlook
While insurers do not yet cover the system for weight management, the cost of Signos subscriptions remains significantly lower than monthly GLP-1 therapies. The company is actively engaging with insurers and employers to secure broader coverage as adoption expands.
As consumer health technologies continue to merge clinical-grade insights with lifestyle applications, Signos’ FDA-cleared CGM platform signals a new era in accessible weight management—one where continuous monitoring, data-driven personalization, and proactive behavior change converge.
Source: CNBC
Prepared by: Next Move Strategy Consulting













Add Comment