Cabergoline Market by Purity Level (Purity < 95%, Purity 95%–98%, Purity > 98%), by Dosage Form {Tablets (0.5 mg, 1 mg, 2 mg), Injectables, Oral Solutions)}, by Application (Anti-Parkinson’s Disease, Hyperprolactinemic Disorders, Others), by End-User (Hospitals, Clinics, Homecare Settings, Research Laboratories, Pharmaceutical Companies) – Global Analysis & Forecast, 2025–2030
Industry Outlook
The global Cabergoline Market size was valued at USD 9.50 million in 2024, and is expected to be valued at USD 10.15 million by the end of 2025. The industry is projected to grow, hitting USD 14.07 million by 2030, with a CAGR of 6.76% between 2025 and 2030.
The market is undergoing notable transformation, driven by rising patient awareness, digital healthcare initiatives, brand differentiation, and increasing demand for high-quality, certified pharmaceutical products. Globally, the need for safe, effective, and traceable Endocrine disorder therapeutics is growing, supported by the expansion of e-pharmacies, telemedicine platforms, and AI-enabled supply chain management. Innovations in drug formulation, sustainable manufacturing, packaging, and traceability are enhancing patient confidence, improving treatment adherence, and reinforcing brand reputation. These trends are reshaping market dynamics, enabling pharmaceutical companies to deliver personalized solutions while meeting the expectations of a more health-conscious and digitally connected patient base.
What are the Key Trends in Market?
What are the Major Technological Trends Shaping the Market?
Technological advancements are transforming production, quality control, and delivery systems. Extended-release and combination formulations improve patient compliance and treatment effectiveness. Automation and IoT integration in manufacturing enhance efficiency, consistency, and reduce operational costs. Additionally, pharmaceutical companies are heavily investing in R&D to optimize bioavailability and develop novel formulations. These innovations collectively enable safer, more effective, and patient-friendly Lactation suppression drugs products, supporting market expansion.
How is Demographic Change Influencing the Market?
Demographic shifts, particularly aging populations, are driving higher prevalence of conditions like hyperprolactinemia and Parkinson’s disease, increasing demand for cabergoline. Moreover, growing healthcare access in emerging markets allows a larger patient base to obtain these treatments. Rising awareness among patients and healthcare providers, coupled with increasing disposable incomes, is further supporting market adoption.
What Role does Government Support Play in Driving Growth in the Market?
Government regulations ensure the safety, quality, and efficacy of cabergoline products through stringent approval processes and pharmaceutical standards. Policies promoting healthcare accessibility, affordability, and drug availability facilitate market growth, especially in low- and middle-income countries. Additionally, initiatives supporting pharmaceutical innovation, R&D, and supply chain transparency further encourage investment and strengthen consumer trust in cabergoline medications.
How are Consumer Preferences Changing in the Market?
Consumers and healthcare providers are increasingly prioritizing high-quality, certified medications. There is growing awareness about cabergoline’s therapeutic benefits, safety profiles, and availability, leading to higher adoption rates. Patients now prefer products that are effective, reliable, and convenient, while healthcare providers emphasize prescribing high-purity, clinically validated formulations. This shift is driving manufacturers to focus on product quality, patient education, and accessibility to meet evolving consumer and clinical needs.
What are the Key Market Drivers, Breakthroughs, and Investment Opportunities that will Shape the Industry in Next Decade?
The global cabergoline market demand is witnessing steady growth, driven by expanding healthcare infrastructure, rising healthcare expenditure, and an aging population prone to chronic conditions such as Parkinson’s disease and hyperprolactinemia. Enhanced hospital networks, specialized clinics, and improved diagnostic facilities increase access to high-quality treatments, particularly in developed countries like Denmark, Germany, and the UK, where healthcare spending is highest. Aging populations in Europe and Asia, including Japan and Italy, further fuel demand due to higher prevalence of endocrine and neurological disorders. Market growth is tempered by the high cost of branded formulations, but opportunities exist through generic, high-purity, and advanced dosage forms that improve accessibility, patient compliance, and therapeutic outcomes globally.
Growth Drivers:
How is Expansion of Healthcare Infrastructure Driving the Market Demand?
The expansion of healthcare infrastructure is a significant driver of the market. As hospitals, clinics, and specialty care centers increase in number and improve in quality, especially in emerging markets, more patients gain access to diagnosis and treatment of conditions like hyperprolactinemia and Parkinson’s disease. Enhanced infrastructure includes better diagnostic facilities, wider availability of pharmacies, and specialized endocrinology and neurology departments, which collectively enable timely prescriptions of Cabergoline pharmaceutical industry. Additionally, improved healthcare networks and increased healthcare spending make it easier for patients to access high-quality medications, thereby boosting overall market demand for cabergoline products globally.
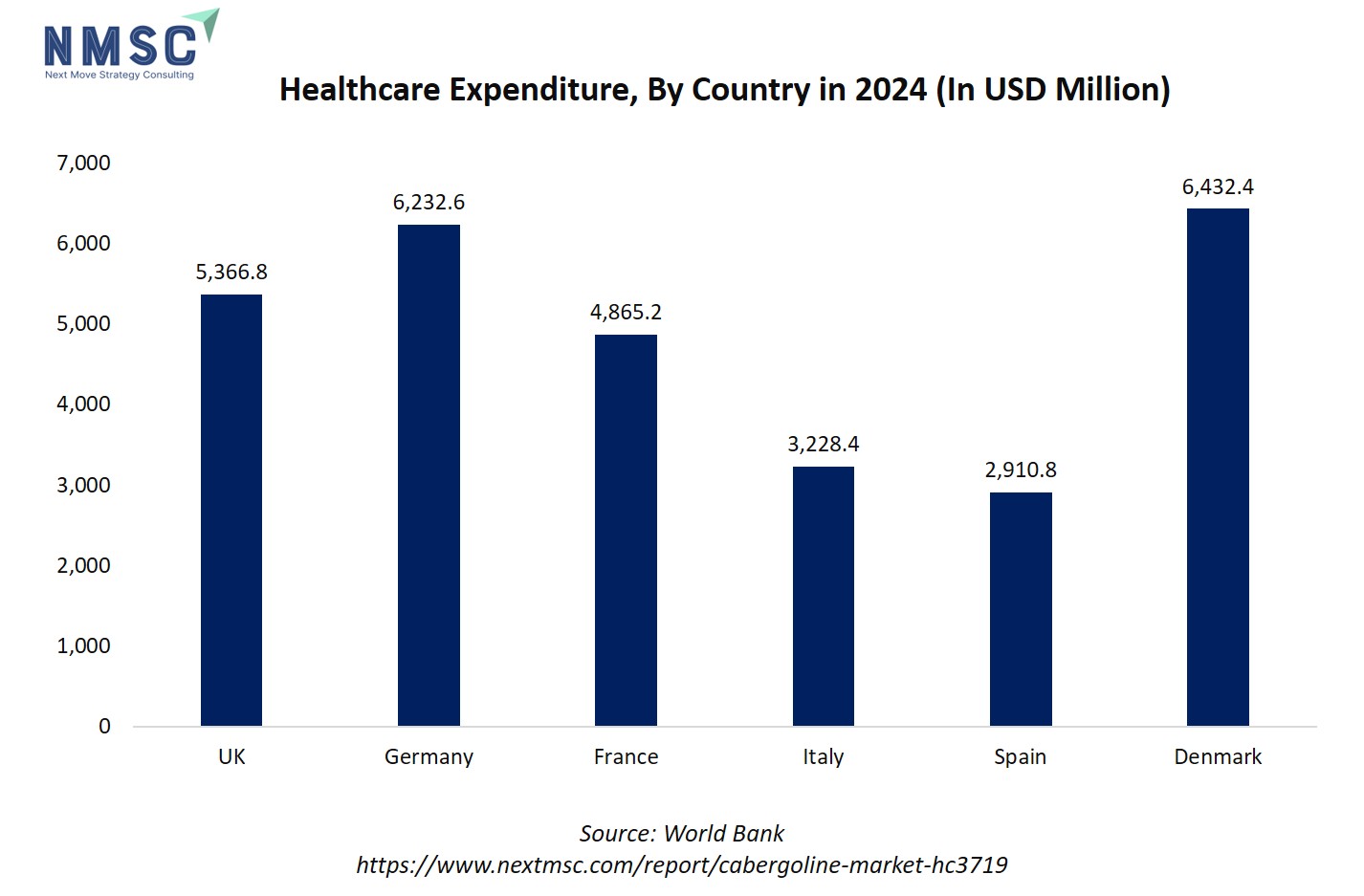
The bar chart illustrating healthcare expenditure by country in 2024 highlights substantial differences in healthcare investment across Europe. Countries like Denmark (6,432.4 million USD), Germany (6,232.6 million USD), and the United Kingdom (5,366.8 million USD), with the highest healthcare spending, are likely to have greater access to advanced treatments and pharmaceuticals, including Cabergoline for chronic conditions such as Parkinson’s disease and hyperprolactinemic disorders. Higher healthcare expenditure supports better diagnostic capabilities, wider availability of specialty medications, and reimbursement policies, which drive adoption in hospitals, clinics, and homecare settings. In contrast, countries with lower healthcare spending, like Spain (2,910.8 million USD) and Italy (3,228.4 million USD), see slower market growth due to limited access, lower treatment penetration, and constrained funding for chronic disease management. Overall, market growth in Europe is closely linked to national healt
Is Aging Population Accelerating the Market Growth?
The aging population is a key driver of the market, as older adults are more prone to conditions such as hyperprolactinemia, Parkinson’s disease, and other endocrine or neurological disorders that require cabergoline treatment. As life expectancy rises globally, the number of elderly patients needing effective therapeutic interventions increases, directly boosting demand for cabergoline. Additionally, aging populations have higher healthcare awareness and greater access to medical services, leading to earlier diagnosis and consistent treatment adherence. This demographic trend ensures sustained market growth, particularly in developed countries with larger elderly populations.
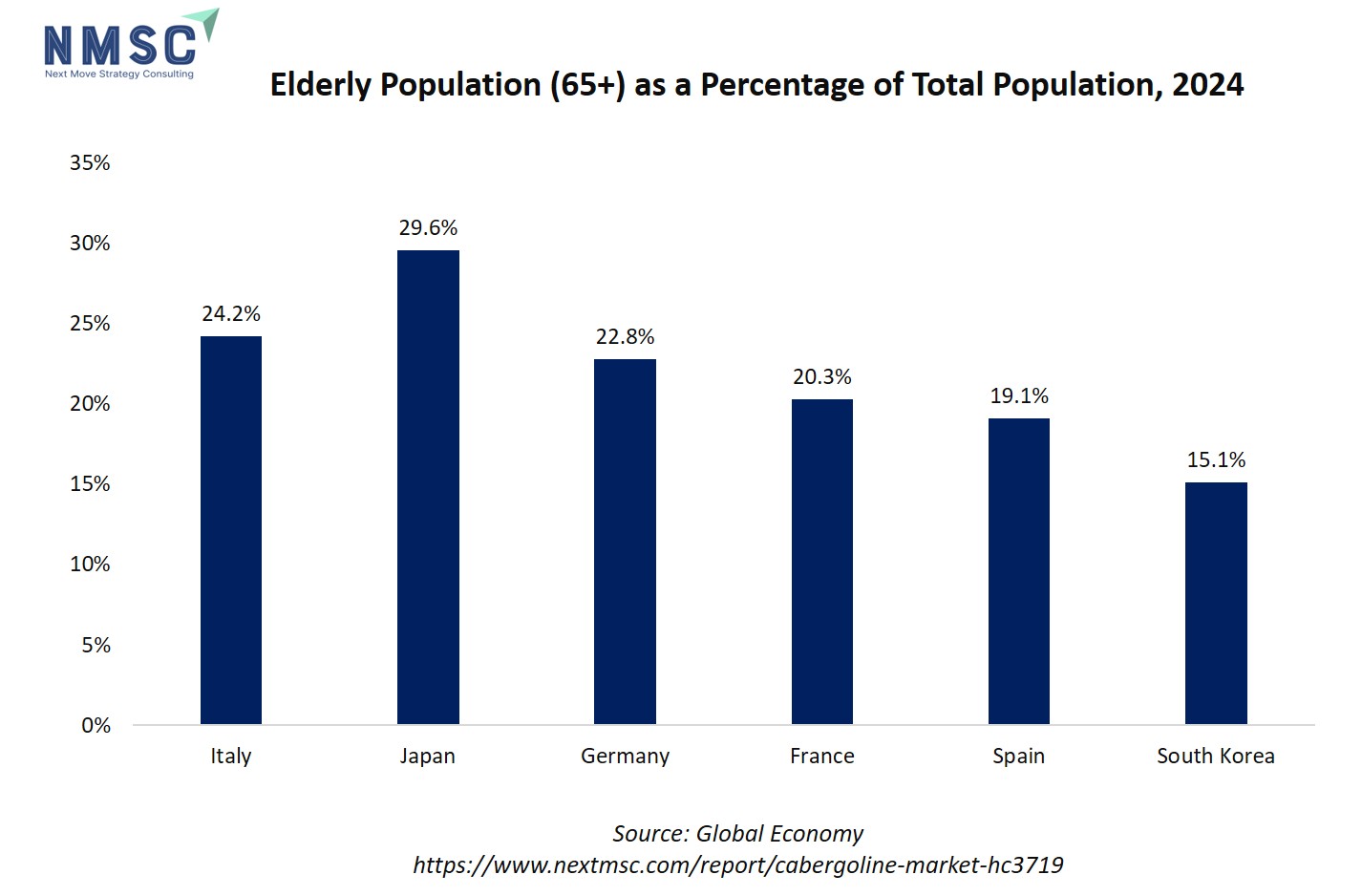
The bar chart illustrates the elderly population (65+) as a percentage of total population in 2024 for selected countries. Japan has the highest proportion at 29.56%, followed by Italy (24.22%), Germany (22.79%), France (20.30%), Spain (19.10%), and South Korea (15.10%). The chart shows that developed nations, particularly in Europe and Asia, are experiencing significant aging populations, with Japan leading the trend.
Growth Inhibitors:
How does High Cost of Branded Cabergoline Products Limit the Growth of the Market?
The relatively high price of branded cabergoline formulations limit adoption, particularly in price-sensitive regions and among patients in emerging markets. Many consumers and healthcare providers prefer generic alternatives, which restrict market growth for premium brands. Additionally, frequent treatment regimens be perceived as a financial burden, further constraining widespread use.
How is Development of Advanced and Generic Formulations Creating Opportunity for the Market?
The market presents significant opportunities for pharmaceutical companies to innovate with extended-release, combination therapies, and high-purity generic cabergoline products. These developments enhance patient compliance, improve therapeutic outcomes, and make treatments more affordable and accessible, particularly in emerging markets. This trend allows manufacturers to expand their market reach and cater to diverse patient needs globally.
How is the Cabergoline Market Report Segmented, and What are the Key Insights from the Segmentation Analysis?
By Purity Level Insights
Which Purity Level is Expected to Drive the Market in 2025?
On the basis of purity level, the market is segmented into Purity < 95%, Purity 95%–98%, and Purity > 98%. The market in 2025 is expected to be primarily driven by the Purity > 98% segment, as this segment meets the stringent clinical and regulatory requirements for pharmaceutical applications and constitutes the majority of total shipments.
Other segments, including Purity 95%–98% and Purity < 95%, continue to maintain relevance in niche applications, research use, and cost-sensitive formulations. Rising focus on high-quality, safe, and effective API production is further boosting demand for the Purity > 98% segment across hospitals, clinics, and pharmaceutical companies.
By Dosage form Insights
Which Dosage form Segment will Lead the Market in 2025?
Analyzing the cabergoline market demand by dosage form, the segments include Tablets (0.5 mg, 1 mg, 2 mg), Injectables, and Oral Solutions. The market in 2025 is expected to be predominantly driven by Tablets, particularly the 1 mg and 2 mg strengths, due to their convenience, patient compliance, and widespread prescription for chronic conditions such as Parkinson’s disease and hormonal disorders.
Injectables and Oral Solutions also hold importance in hospital and specialized clinical settings, offering flexibility for patients who cannot take oral tablets. Growing patient preference for easy-to-administer, standardized doses is further supporting the growth of the tablet segment across key regions.
By Application Insights
Which Therapeutic Application will Drive the Cabergoline Market Growth in 2025?
The market is segmented by therapeutic application into Anti-Parkinson’s Disease, Hyperprolactinemic Disorders, and Others. In 2025, the market is expected to be primarily driven by Anti-Parkinson’s Disease, owing to the rising prevalence of age-related neurological disorders, increasing diagnosis rates, and strong physician preference for Cabergoline as a first-line treatment.
The Hyperprolactinemic Disorders segment continues to grow steadily, supported by the drug’s effectiveness in managing hormonal imbalances and reproductive health issues. The “Others” category, including niche endocrine and age-related conditions, is also gaining traction due to expanding clinical research and patient awareness initiatives.
By End-User Insights
Which End-user Segment is Poised to Lead the Cabergoline Market in 2025?
The market is segmented by end-user into Hospitals, Clinics, Homecare Settings, Research Laboratories, and Pharmaceutical Companies. In 2025, Hospitals are expected to dominate the market due to their role in treating chronic and complex conditions such as Parkinson’s disease and hyperprolactinemic disorders, which require professional supervision and regular monitoring.
Clinics also contribute significantly, particularly for outpatient treatments and follow-up therapies, while Homecare Settings are gaining traction as patient-centric care models expand. Research Laboratories and Pharmaceutical Companies play a vital role in drug development, clinical trials, and large-scale manufacturing, supporting the overall growth of the market across regions.
Regional Outlook
The market is geographically studied across North America, Europe, Asia Pacific, and the Middle East & Africa, and each region is further studied across countries.
Cabergoline Market in North America
The North American’s market is driven by increasing awareness of endocrine and neurological disorders, growing diagnosis rates, and expansion of healthcare infrastructure. Rising adoption of advanced formulations and generic options enhances accessibility, while urbanization and higher disposable incomes support demand for effective treatment solutions. Digital healthcare platforms and telemedicine services are improving patient engagement, prescription management, and home delivery of medications, further strengthening market growth in the region.
Cabergoline Market in the United States
In the U.S., market growth is primarily driven by high prevalence of hyperprolactinemia, Parkinson’s disease, and related conditions. Increasing physician awareness, early diagnosis, and patient education initiatives are fueling demand for both branded and generic cabergoline. Telehealth and e-prescription platforms improve accessibility, while rising disposable incomes and advanced healthcare facilities encourage adoption of effective therapeutic solutions.
Cabergoline Market in Canada
Canada’s market is expanding due to improved healthcare access, increasing patient awareness, and availability of advanced and generic cabergoline formulations. Urbanization and rising healthcare spending drive adoption among patients with endocrine and neurological disorders. Telemedicine, digital prescriptions, and pharmacy networks are enhancing convenience and distribution, supporting overall market growth.
Cabergoline Market in Europe
The European market is supported by rising prevalence of target diseases, well-established healthcare systems, and strong regulatory frameworks ensuring drug safety and efficacy. Advanced research initiatives, patient education programs, and growing awareness of treatment options are boosting adoption. Urbanization, higher disposable incomes, and technologically advanced healthcare services are further driving market expansion.
Cabergoline Market in the United Kingdom
In the U.K., growth is driven by increased diagnosis of hyperprolactinemia and Parkinson’s disease, combined with high patient awareness and access to advanced treatment options. Healthcare digitization, telemedicine, and widespread pharmacy networks improve accessibility. Rising disposable incomes and urban lifestyles support the adoption of both branded and generic cabergoline products, while ongoing R&D ensures innovative treatment options are available.
Cabergoline Market in Germany
Germany’s market is growing due to high prevalence of target disorders, advanced healthcare infrastructure, and strong patient awareness programs. Easy access to pharmacies, telemedicine services, and widespread availability of generic cabergoline enhances adoption. Increased urbanization, higher disposable incomes, and focus on preventive healthcare contribute to steady market expansion.
Cabergoline Market in France
In France, the market is supported by increased awareness and early diagnosis of endocrine and neurological disorders. High-quality healthcare services, availability of generic options, and patient education campaigns encourage uptake of cabergoline. Urbanization and rising healthcare expenditure support access to effective treatment, while ongoing research and clinical studies drive innovation in formulations and delivery methods.
Cabergoline Market in Spain
Spain’s market is influenced by growing prevalence of hyperprolactinemia and Parkinson’s disease, improving healthcare infrastructure, and rising patient awareness. Urbanization, increasing healthcare spending, and telemedicine adoption facilitate access to cabergoline. Availability of generic and advanced formulations, combined with physician education and awareness campaigns, is driving growth and expanding treatment coverage across the country.
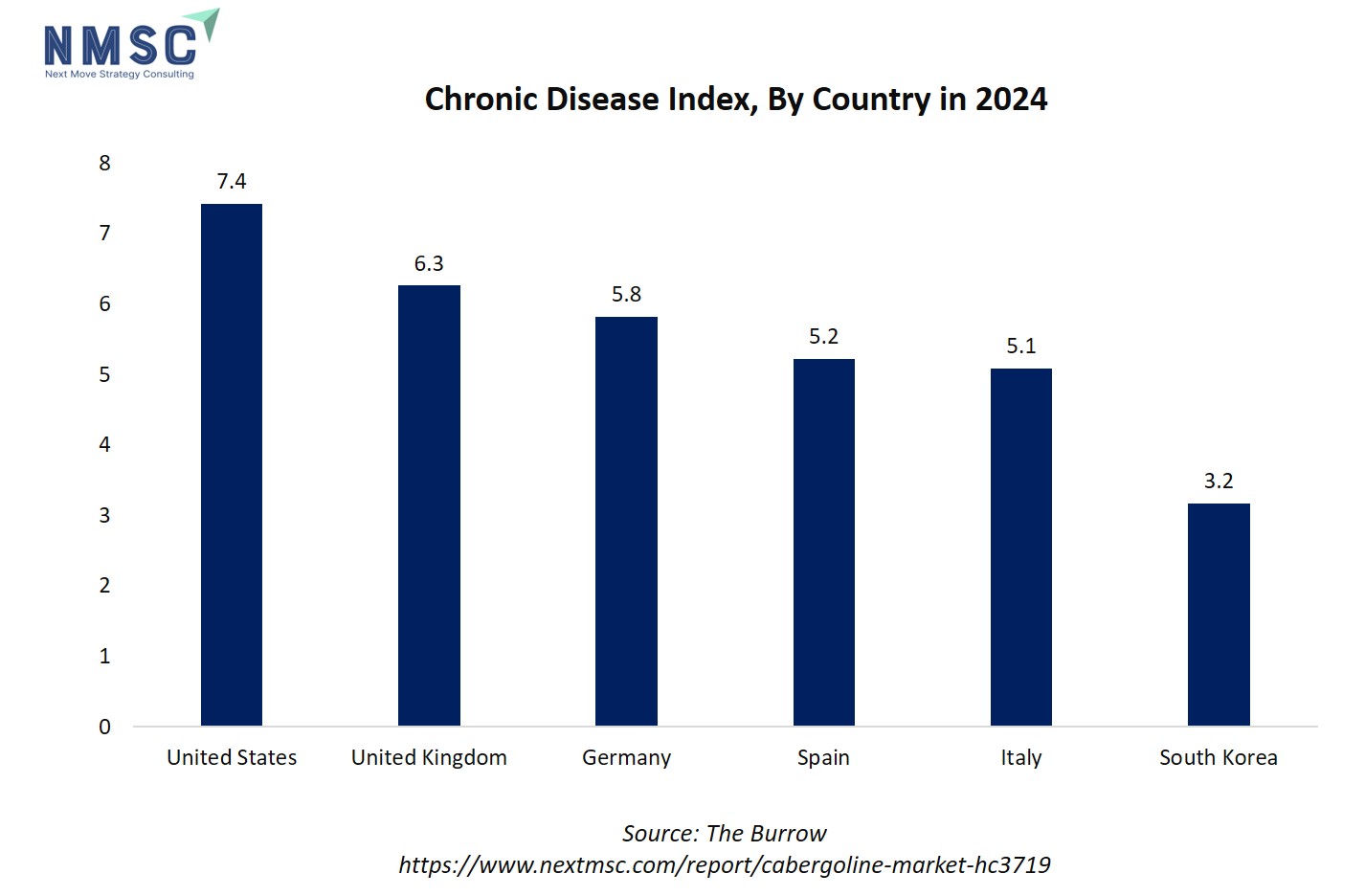
The bar chart illustrates the Chronic Disease Index for selected countries in 2024. The United States has the highest index at 7.42, followed by the United Kingdom (6.26), Germany (5.82), Spain (5.22), and Italy (5.09). South Korea has the lowest index at 3.17, indicating a relatively lower prevalence of chronic conditions. The chart highlights that North America and Western Europe have a higher burden of chronic diseases compared to Asian countries like South Korea.
Cabergoline Market in Italy
Italy’s market growth is supported by increasing healthcare digitization, expanding access to pharmacies, and adoption of telemedicine. Rising awareness of endocrine and neurological disorders, combined with higher disposable incomes and urbanization, is driving adoption of cabergoline for effective treatment. Advanced formulations and generic options further improve accessibility, while healthcare campaigns and patient education promote proper use, contributing to steady market growth.
Cabergoline Market in the Nordics
In Nordic countries, growth is driven by advanced healthcare infrastructure, high awareness of endocrine and neurological disorders, and widespread adoption of telehealth services. Urbanization and digital health platforms facilitate patient access to cabergoline, while aging populations and higher healthcare spending support consistent market demand across the region.
Cabergoline Market in Asia Pacific
The Asia Pacific’s market is expanding rapidly due to rising prevalence of target conditions, increasing healthcare access, and growing awareness among patients. Urbanization, rising disposable incomes, and adoption of digital healthcare platforms support the distribution and accessibility of cabergoline. Countries like China, India, South Korea, and Indonesia are showing strong demand for both branded and generic formulations, with healthcare digitization enhancing patient engagement and prescription management.
Cabergoline Market in China
China’s market is primarily driven by rapid urbanization, increasing patient awareness, and expanding healthcare infrastructure. Digital healthcare platforms, telemedicine, and e-pharmacy services are improving accessibility to cabergoline. Rising disposable incomes and emphasis on advanced treatment options further fuel demand for high-quality and generic formulations.
Cabergoline Market in Japan
Japan’s growth is influenced by aging populations, high digital adoption in healthcare, and increasing patient awareness of neurological and endocrine disorders. Telehealth services and strong pharmacy networks enhance access to cabergoline. Rising healthcare expenditure and preference for effective treatment options contribute to steady market expansion.
Cabergoline Market in India
India’s market is driven by improving healthcare infrastructure, urbanization, and increasing awareness of endocrine and neurological conditions. Expansion of digital healthcare platforms, telemedicine, and pharmacy networks enhances access to cabergoline. Rising disposable incomes, growing patient education, and adoption of generic and advanced formulations create strong growth opportunities.
Cabergoline Market in South Korea
South Korea’s market is fueled by high awareness of disease management, advanced healthcare systems, and telemedicine adoption. Urbanization and digital healthcare services improve patient access to cabergoline. Growing demand for effective treatment, supported by strong patient education and physician awareness, sustains market growth.
Cabergoline Market in Taiwan
Taiwan’s growth is supported by well-developed healthcare infrastructure, increasing patient awareness, and digital healthcare adoption. Urbanization and rising healthcare spending facilitate access to cabergoline. Initiatives in patient education, availability of generic and advanced formulations, and improved distribution channels drive steady market expansion.
Cabergoline Market in Indonesia
Indonesia’s market is driven by rapid urbanization, growing healthcare access, and increasing awareness of endocrine and neurological disorders. Expansion of hospitals, clinics, and digital healthcare platforms improves availability of cabergoline. Rising disposable incomes, adoption of advanced and generic formulations, and patient education programs support market growth across urban and semi-urban regions.
Cabergoline Market in Australia
Australia’s market growth is supported by well-developed healthcare infrastructure, digital health adoption, and high patient awareness. Telemedicine services, pharmacy networks, and home delivery options enhance accessibility to cabergoline. Rising urbanization, increasing healthcare expenditure, and demand for effective treatment solutions contribute to the steady expansion of the market.
Cabergoline Market in Latin America
In Latin America, the market is growing due to expanding healthcare infrastructure, urbanization, and increasing patient awareness of neurological and endocrine disorders. Countries such as Brazil, Mexico, and Argentina are seeing higher demand for cabergoline, supported by improved pharmacy networks, telemedicine, and access to generic and advanced formulations. Rising disposable incomes and education about treatment options are further driving market adoption.
Cabergoline Market in the Middle East & Africa
The cabergoline market challenges in the Middle East and Africa is supported by improving healthcare infrastructure, increasing urbanization, and higher patient awareness. Expansion of clinics, hospitals, and pharmacy networks enhances accessibility. Rising disposable incomes, adoption of generic and advanced formulations, and focus on patient education contribute to steady growth in both urban and semi-urban regions.
Competitive Landscape
Which Companies Dominate the Cabergoline Market and How do they Compete?
The global cabergoline market share is dominated by a mix of multinational pharmaceutical giants, regional leaders, and specialized producers, including Pfizer Inc., Sun Pharmaceutical Industries Ltd., Somerset Therapeutics, LLC, Viatris, Teva Pharmaceutical Industries Ltd., Actiza Pharmaceuticals Pvt. Ltd., Advacare Pharma, Weefsel Pharma, Global Calcium Pvt. Ltd., Cobalt Pharmaceuticals Company, Ingenus Pharmaceuticals, CF Pharma Ltd., Yonsung Fine Chem., Finetech Pharma, and Lifecare Laboratories Pvt. Ltd. These companies compete by offering a wide range of Cabergoline products, including API (active pharmaceutical ingredient), tablets, and other formulations targeting conditions such as hyperprolactinemia and Parkinson’s disease. Market leaders differentiate themselves through high product quality, regulatory compliance (USFDA DMF, EU-GMP, WHO-GMP), innovation in formulations, and patient-friendly dosage forms. They also leverage strong global and regional distribution networks, ensuring availability in key markets while maintaining competitive pricing.
Smaller and specialized producers focus on niche markets, high-purity API supply, and regional distribution, enabling them to serve manufacturers and generic formulators effectively. Companies that combine regulatory compliance, operational efficiency, product innovation, and cost-effective production capture significant market share and foster strong partnerships with healthcare providers and distributors. In this competitive landscape, Pfizer, Teva, and Viatris dominate branded and generic formulations, while Global Calcium, CF Pharma, Yonsung Fine Chem, Finetech Pharma, and Lifecare Laboratories play pivotal roles as API manufacturers, together shaping a market where quality, innovation, and reliability are the key drivers of success.
Market Dominated by Cabergoline Market Giants and Specialists
The market is led by a combination of large multinational pharmaceutical companies and regional or niche manufacturers. Major players compete on product quality, brand reputation, R&D capabilities, and distribution networks, offering a wide portfolio of cabergoline formulations, including branded and generic products. Smaller or specialized firms focus on niche therapeutic areas, advanced drug delivery systems, or cost-effective generics, catering to specific patient populations. This dual market structure allows healthcare providers and patients to choose between established brands for reliability or specialized firms for innovative and targeted treatment options.
Innovation and Adaptability Drive Market Success
Leading cabergoline manufacturers invest heavily in R&D to enhance drug efficacy, bioavailability, and patient compliance. Innovations include extended-release formulations, combination therapies, and advanced delivery mechanisms. Companies adopting digital tools for patient education, telemedicine integration, and supply chain optimization strengthen market presence. Those focusing on personalized treatment options, high-quality generics, and innovative packaging maintain competitive advantage and respond effectively to evolving patient and healthcare provider needs.
Market Players to OPT for Merger & Acquisition Strategies to Expand their Presence
M&A activities are increasingly adopted by cabergoline manufacturers to expand product portfolios, enter new geographic markets, and acquire specialized R&D capabilities. Leading pharmaceutical firms acquire niche or regional players to scale operations, diversify offerings, and integrate advanced drug technologies. These strategies enable companies to capture new patient segments, respond to increasing demand for high-quality therapeutic solutions, and strengthen their competitive positioning in both established and emerging markets.
List of Key Cabergoline Companies
-
Pfizer Inc.
-
Sun Pharmaceutical Industries Ltd.
-
Somerset Therapeutics, LLC
-
Viatris
-
Teva Pharmaceutical Industries Ltd.
-
Actiza Pharmaceuticals Pvt. Ltd.
-
Advacare Pharma
-
Weefsel Pharma
-
Global Calcium Pvt. Ltd.
-
Cobalt Pharmaceuticals Company
-
Ingenus Pharmaceuticals
-
CF Pharma Ltd.
-
Yonsung Fine Chem.
-
Finetech Pharma
-
Lifecare Laboratories Pvt. Ltd.
What are the Latest Key Industry Developments?
-
July 2025 - Teva reported stronger-than-expected second-quarter profits for 2025, driven by a 26% increase in sales of branded drugs used to treat migraines, Huntington's disease, and schizophrenia.
-
September 2025 - Pfizer is reinforcing its presence in the obesity treatment market through a USD 4.9 billion acquisition of Metsera Inc., a development-stage drug company with four drug programs in clinical development.
What are the Key Factors Influencing Investment Analysis & Opportunities in the Cabergoline Market?
The market is attracting significant investor interest due to the rising global demand for high-quality, effective, and diverse therapeutic products. Companies offering a broad portfolio of cabergoline formulations, including branded, generic, and advanced extended-release options, are particularly appealing, as strong R&D capabilities enhance efficacy, bioavailability, and patient compliance. Advanced manufacturing processes, stringent quality control, and efficient supply chain management further strengthen investor confidence. Firms with extensive regional and international distribution networks, as well as those pursuing strategic initiatives such as mergers, acquisitions, and partnerships, demonstrate strong growth potential. Compliance with regulatory standards, adaptability to evolving healthcare needs, and sustainable practices make these companies prime candidates for investment in the Cabergoline sector.
Key Benefits for Stakeholders:
Next Move Strategy Consulting (NMSC) presents a comprehensive analysis of the Cabergoline Market forecast, covering historical trends from 2020 to 2024 and providing detailed forecasts through 2030. The report examines the market at both regional and country levels, offering quantitative insights into key growth drivers, challenges, and investment opportunities across all major therapeutic, dosage form, and distribution segments of the Cabergoline industry.
The cabergoline market analysis offers considerable benefits to a wide range of stakeholders by addressing the growing global demand for effective treatments for hormonal disorders such as hyperprolactinemia and Parkinson’s disease. Investors benefit from strong growth potential driven by rising disease prevalence, increasing awareness of endocrine health, and expanding access to affordable generic formulations. Healthcare providers and pharmaceutical companies gain from Cabergoline’s proven efficacy, favorable safety profile, and extended therapeutic applications, which enhance patient outcomes and treatment adherence. Manufacturers and suppliers enjoy opportunities for product diversification, dosage innovation, and market expansion, particularly in emerging economies with developing healthcare systems. Additionally, regulatory agencies and policymakers support Cabergoline adoption through efforts to improve access to cost-effective therapies and promote innovation in endocrine and neurological care. This
Report Scope:
|
Parameters |
Details |
|
Market Size in 2025 |
USD 10.15 Million |
|
Revenue Forecast in 2030 |
USD 14.07 Million |
|
Growth Rate |
CAGR of 6.76% from 2025 to 2030 |
|
Analysis Period |
2024–2030 |
|
Base Year Considered |
2024 |
|
Forecast Period |
2025–2030 |
|
Market Size Estimation |
Billion (USD) |
|
Growth Factors |
|
|
Companies Profiled |
15 |
|
Countries Covered |
33 |
|
Market Share |
Available for 10 companies |
|
Customization Scope |
Free customization (equivalent to up to 80 analyst-working hours) after purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and Purchase Options |
Avail customized purchase options to meet your exact research needs. |
|
Approach |
In-depth primary and secondary research; proprietary databases; rigorous quality control and validation measures. |
|
Analytical Tools |
Porter's Five Forces, SWOT, value chain, and Harvey ball analysis to assess competitive intensity, stakeholder roles, and relative impact of key factors. |
Key Market Segments
By Purity Level
-
Purity < 95%
-
Purity 95%–98%
-
Purity > 98%
By Dosage Form
-
Tablets
-
0.5 mg
-
1 mg
-
2 mg
-
-
Injectables
-
Oral Solutions
By Application
-
Anti-Parkinson’s Disease
-
Hyperprolactinemic Disorders
-
Others
By End-User
-
Hospitals
-
Clinics
-
Homecare Settings
-
Research Laboratories
-
Pharmaceutical Companies
Geographical Breakdown
-
North America: U.S., Canada, and Mexico.
-
Europe: U.K., Germany, France, Italy, Spain, Sweden, Denmark, Finland, Netherlands, and rest of Europe.
-
Asia Pacific: China, India, Japan, South Korea, Taiwan, Indonesia, Vietnam, Australia, Philippines, Malaysia and rest of APAC.
-
Middle East & Africa (MEA): Saudi Arabia, UAE, Egypt, Israel, Turkey, Nigeria, South Africa, and rest of MEA.
-
Latin America: Brazil, Argentina, Chile, Colombia, and rest of LATAM.
Conclusion & Recommendations
The Cabergoline Market segmentation is poised for steady growth as the prevalence of hormonal disorders and neurological conditions continues to rise globally. This report provides stakeholders, investors, and healthcare providers with actionable insights to capitalize on emerging opportunities in endocrine and neurological therapeutics. Increasing awareness of prolactin-related disorders, expanding treatment adoption for Parkinson’s disease, and growing accessibility of generic formulations are key factors driving market expansion across developed and emerging economies. NMSC’s latest report integrates robust data analytics and strategic frameworks to help organizations navigate shifting clinical trends, regulatory changes, and competitive pressures.
Strategic priorities for market participants include investing in research and development to explore new therapeutic applications, improving drug affordability through generic production, and expanding distribution networks in high-demand regions. Companies focusing on patient-centric care, regulatory compliance, and innovation in dosage forms will enhance treatment outcomes and strengthen market positioning. For executives and investors, success lies in targeting high-growth regions and therapeutic areas with unmet medical needs, such as reproductive health and neurology. Collaborating with healthcare institutions, research organizations, and regional distributors can accelerate drug adoption and improve accessibility. Additionally, emphasizing pharmacovigilance, ethical marketing, and global supply chain efficiency enhances brand credibility and patient trust. By prioritizing innovation, accessibility, and clinical excellence, organizations can unlock long-term value and establish a strong competitive pos

















 Speak to Our Analyst
Speak to Our Analyst